Dr. Grzegorz Grabe has been awarded the POLONEZ Bis 2 grant by National Science Centre, Poland. His project entitled Dynamics and evolution of specificity of TA complexes will be carried out at our lab MRSLab at the Intercollegiate Faculty of Biotechnology of the University of Gdansk and Medical University of Gdansk. Looking forward to our collaboration on this exciting project!
Grzegorz Grabe received his BSc (2007) and MSc (2010) degrees at Intercollegiate Faculty of Biotechnology in University of Gdansk and Medical University of Gdansk. In 2011 he was awarded the Wellcome Trust MRes+PhD studentship that he undertook at Imperial College London in a research group of Professor David Holden. While there, he studied virulence of an intracellular bacterial pathogen, Salmonella enterica. Having obtained his PhD degree in 2016, he then joined the lab of Professor Sophie Helaine at Imperial College London and later Harvard Medical School to study mechanisms of neutralization and regulation of Salmonella enterica toxin-antitoxin systems.
The POLONEZ BIS is another attractive grants for researchers coming from outside Poland. It is aimed at people who have a doctoral degree or at least four years of full-time research experience, and who have not resided, been employed or studied in Poland for more than a total of 12 months in the three years preceding the opening date of the competition. POLONEZ BIS is co-financed by the European Commission and the National Science Center under the Marie Skłodowska-Curie COFUND grant.
Project description
Toxin-antitoxin (TA) systems are ubiquitous genetic modules promoting bacterial survival by regulation and arrest of cell growth in response to stresses such as phage infection, antibiotic exposure, or macrophage uptake. While toxins target essential biological processes affecting growth of a cell, antitoxins specifically neutralize their cognate toxins and allow for cell growth. In the most prevalent type II TAs, the antitoxin is a protein that directly binds its cognate toxin with a neutralization domain (ND), resulting in formation of a tight toxin-antitoxin complex. Besides the ND, the antitoxin also contains a DNA binding domain enabling it to transcriptionally control the toxin-antitoxin operon. Another key feature of TAs is the common presence of several paralogues of the same system within the same organism. The frequent complete insulation of numerous paralogous TAs, where there is high specificity with no cross-pairing, suggests that a neutralizing crossinteraction is detrimental to the cell.
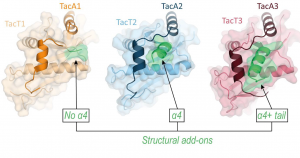
I am interested in studying the evolution of specificity of paralogous TA systems through toxin size variation by addition of structural add-on elements then allowing the antitoxin to evolve a specific neutralizing sequence. I found that such size alteration occurs at either N- or C-terminal toxin ends in various TA systems. Building on the experience gained while studying three paralogous Salmonella TacAT1-3 TA systems, I will continue to explore the significance of add-ons in contribution to development of neutralization specificity reducing paralogue cross-talk conflict. I will also investigate the activation of an important family of TAs involved in persistence of pathogenic Salmonella. Overall, these studies will provide new insights into the dynamics of evolution of paralogous protein-protein complexes and protein-DNA complexes, thereby opening avenues of interfering with these important stress response elements in bacteria.